2H2 + O2 → 2H2O
H2 + F2 → 2HF
2K + H2 → 2KH
2Na + H2 → 2NaH
Ca + H2 → CaH2
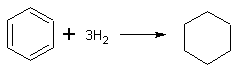
C6H5CH3 + 3H2 → C6H11CH3
C6H5OH + 3H2 → C6H11OH
CuO + H2 → Cu + H2O
MoO3 + 3H2 → Mo + 3H2O
CH3CH2CH2CH2CH=CH2 + H2 → CH3(CH2)4CH3
CH3CHBrCH2CH2CH3 + Mg → CH3CH(MgBr)CH2CH2CH3
CH3CH(MgBr)CH2CH2CH3 + H2SO4 → CH3CH2CH2CH2CH3 + MgSO4 + HBr
NOCl + 3H2 → NH4Cl + H2O
2NOCl + 3H2 → N2 + 2H2O + 2HCl
2C + H2 → C2H2
C6H5Na + H2 → C6H6 + NaH
CO + 3H2 → CH4 + H2O
S2Cl2 + H2 → 2S + 2HCl
S + H2 → H2S
VCl4 + H2 → VCl2 + 2HCl
2Na2O + H2 → 2NaOH + 2Na
N2 + 3H2 → 2NH3
H2 + PdCl2 → Pd + 2HCl
H2 + I2 → 2HI
H2 + Cl2 → 2HCl
C2H5OOC2H5 + H2 → 2C2H5OH
(CH3)3COOC(CH3)3 + H2 → 2(CH3)3COH
2CuCl + H2 → 2Cu + 2HCl
2NOCl + H2 → 2HCl + 2NO
H2 + Br2 → 2HBr
((CH3)3C6H2)2PC6F4B(C6F5)2 + H2 → ((CH3)3C6H2)2PHC6F4BH(C6F5)2
Fe2O3 + 3H2 → 2Fe + 3H2O
Sb2O3 + 3H2 → 2Sb + 3H2O
2TiBr4 + H2 → 2TiBr3 + 2HBr
SrSO4 + 4H2 → SrS + 4H2O
2CrCl3 + H2 → 2CrCl2 + 2HCl
2ReCl3 + 3H2 → 2Re + 6HCl
CH2=CH2 + H2 → C2H6