Main page (Russian)
 Search in database (English)
Search in database (English)
Properties of substance:
N-[2-[(2,6-dimethylphenyl)amino]-2-oxoethyl]-N,N-diethylbenzenemethanaminium benzoate
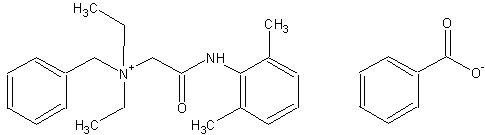
skc-fileSynonyms:
denatonium benzoate
bitrex
Group of substances:
organic
Physical appearance:
colorless powderEmpirical formula (Hill's system for organic substances):
C28H34N2O3Structural formula as text:
C6H3(CH3)2NHCOCH2N(C2H5)2CH2C6H5(C6H5COO)Molar/atomic mass: 446.59
Melting point (°C):
166-174CAS ¹: 3734-33-6
Solubility (g/100 g of solvent):
2-propanol: insoluble [Ref.]
acetone: sparingly soluble [Ref.]
acetonitrile: insoluble [Ref.]
chloroform: 33 (20°C) [Ref.]
diethyl ether: 0.028 (20°C) [Ref.]
dimethylsulfoxide: moderately soluble [Ref.]
ethanol: 50 (20°C) [Ref.]
ethyl acetate: insoluble [Ref.]
hexane: insoluble [Ref.]
methanol: very soluble [Ref.]
toluene: insoluble [Ref.]
water: 5 (20°C) [Ref.]
Hygroscopic:
yes
Numerical data:
Bitter taste threshold concentrations (mM): 4.16E-6
Synthesis 1:
Reference: Stachowiak W., Wysocki M., Niemczak M. “Bitter” Results: Toward Sustainable Synthesis of the Most Bitter Substances, Denatonium Saccharinate and Denatonium Benzoate, Starting from a Popular Anesthetic, Lidocaine / Journal of Chemical Education. - 2022. - Vol. 99, No. 4 pp. 1606, S14 [doi: 10.1021/acs.jchemed.1c00995](CH3)2C6H3NHCOCH2N(C2H5)2(CH2C6H5)Cl + NaOH → (CH3)2C6H3NHCOCH2N(C2H5)2(CH2C6H5)OH + NaCl
(CH3)2C6H3NHCOCH2N(C2H5)2(CH2C6H5)OH + C6H5COOH → (CH3)2C6H3NHCOCH2N(C2H5)2(CH2C6H5)(C6H5COO) + H2O
Time needed: 4 hours
Introduce 1.30 g (3.6 mmol) of denatonium chloride, 5 ml of commercial ethanol with 0.20 g (3.6 mmol) of potassium hydroxide to the round-bottom flask. Mix the content of the flask for 10 minutes and then cool it to the temperature of 4°C. Then, separate the precipitated potassium chloride via vacuum filtration, wash the sediment with 2 ml of cold ethanol. Subsequently, neutralize filtrate with 0.44 g (3.6 mmol) of benzoic acid (Note: Water-moistened test strip should be used to detect the end of neutralization). In the next step, evaporate the solvent from solution under reduced pressure in the rotary evaporator. As a result, 1.55 g (96%) of white solid, characterized by melting point in a range from 164.0 to 168.0°C should be obtained.
LD50 (mg/kg):
612 (rats, oral)
References:
- Catalog handbook of fine chemicals Aldrich, 1992-1993. - pp. 133
- Clarke's Analysis of Drugs and Poisons. - 4 ed. - London, 2011. - pp. 1203
- Remington: The Science and Practice of Pharmacy. - 21st ed. - Lippincott Williams and Wilkins, 2005. - pp. 1085
- Schlager N., Weisblatt J., Newton D.E. Chemical Compounds. - 2006. - pp. 271-275
- The Merck Index 11th ed., Merck & Company, 1989. - pp. 454-455
- The Merck Index. - 15th ed. - Royal Society of Chemistry, 2013. - pp. 523
- Ïàòåíò ÑØÀ US5,891,919 (îò 06.04.1999)
Your requests if no data into database
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru

